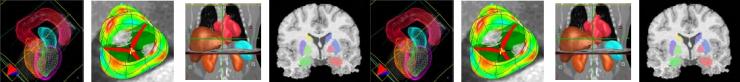
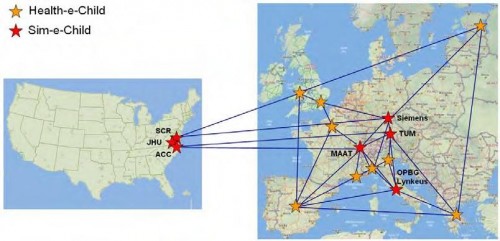
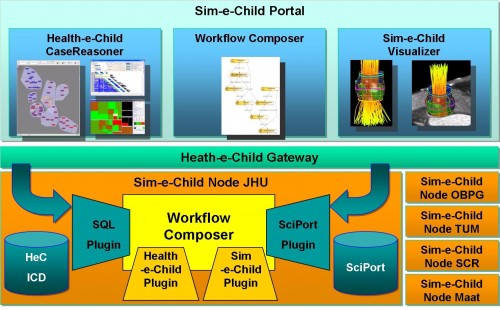
The FP7 Sim-e-Child project started work in January 2010 as a follow-up to the FP6 Health-e-Child IP. As an early member of the Virtual Physiological Human research community, the Health-e-Child project (www.health-e-child.org) worked for over 4 years from 2006 to build an integrated healthcare platform for paediatrics. The Health-e-Child platform is based on the EGEE gLite grid middleware to integrate innovative predictive disease models, complex data visualization and knowledge discovery applications, with the ultimate goal of supporting clinical decision making in rare paediatrics diseases for three disease areas (congenital heart disease, arthritis and brain tumours in children). The Health-e-Child platform and its applications have won several prizes (including the ICT’08 Exhibit Grand Prize, the EGEE’08 Best Live Demonstration Award, the HealthGrid’08 Best Demonstration Award, the 2008 Medical Informatics Europe Best Poster Award) and are now available over a network of four hospitals in Europe. Now in the early stages of the Sim-e-Child project, the Health-e-Child Grid is deploying at two locations in the US at Johns Hopkins Children’s Centre in Baltimore and Siemens Corporate Research in Princeton. In the coming months Health-e-Child’s platform will be developed to enable it to be used for large scale simulations in paediatric cardiology and as a collaborative environment for constructing and validating multi-scale and personalized models of a growing heart and vessels. Additionally the project will further develop pan-Atlantic cooperation by linking the platform with leading institutions such as the American College of Cardiology and the Technical University of Munich.
Sim-e-Child has three primary goals which this expanded Grid network will support:
1. The enhancing of cardiac models by utilising international collaboration beyond the European research area to validate the models on additional data. With the support of the American College of Cardiology and Johns Hopkins,Sim-e-Child is validating Health-e-Child’s heart modelling capabilities using ongoing clinical trial databases (the Coarctation Of the Aorta Stent Trial [COAST] and the National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions [GenTAC]) in collaboration with one of Health-e-Child’s clinical partners, the I.R.C.C.S. Bambino Gesù in Italy.
2. Secondly, the Health-e-Child models are being expanded by integrating existing Siemens Corporate Research models of the aorta, aortic valve and mitral valve together with blood flow modelling and flow visualization from the Technical University of Munich. The new and comprehensive heart model will be applied to congenital aortic disease, thus enriching the portfolio of applications available in Health-e-Child and broadening its end-user community.